FDA Grants Breakthrough Designation to 4D Path for Novel Cancer Diagnostic Solution
Potential for a New Standard of Care to Improve Diagnostic Accuracy for Breast Cancer Exclusively from H&E Biopsy/Resection Images
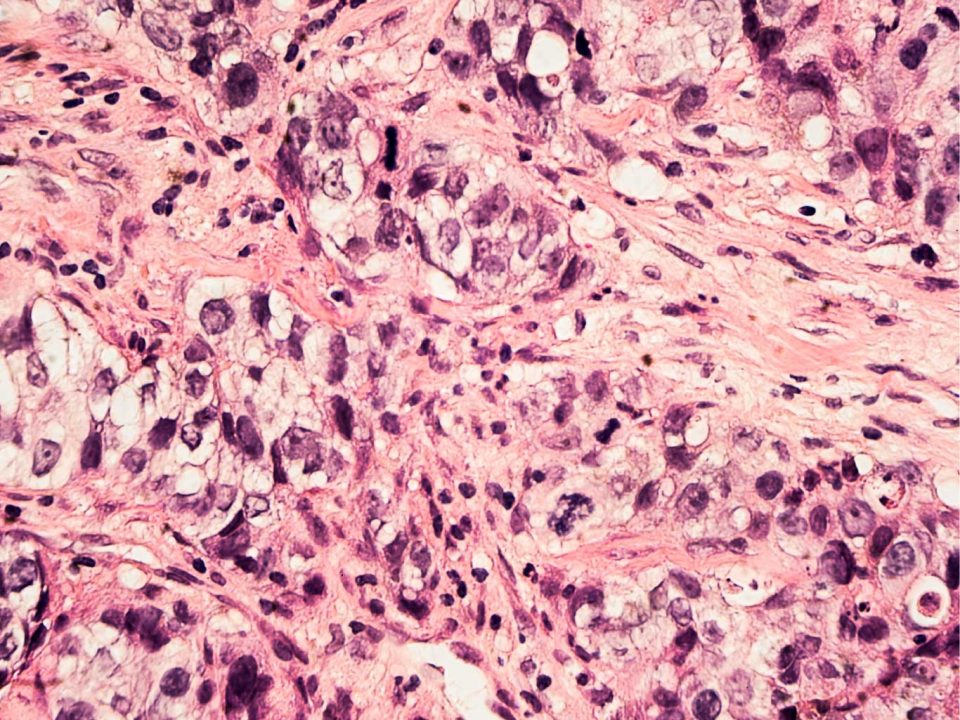
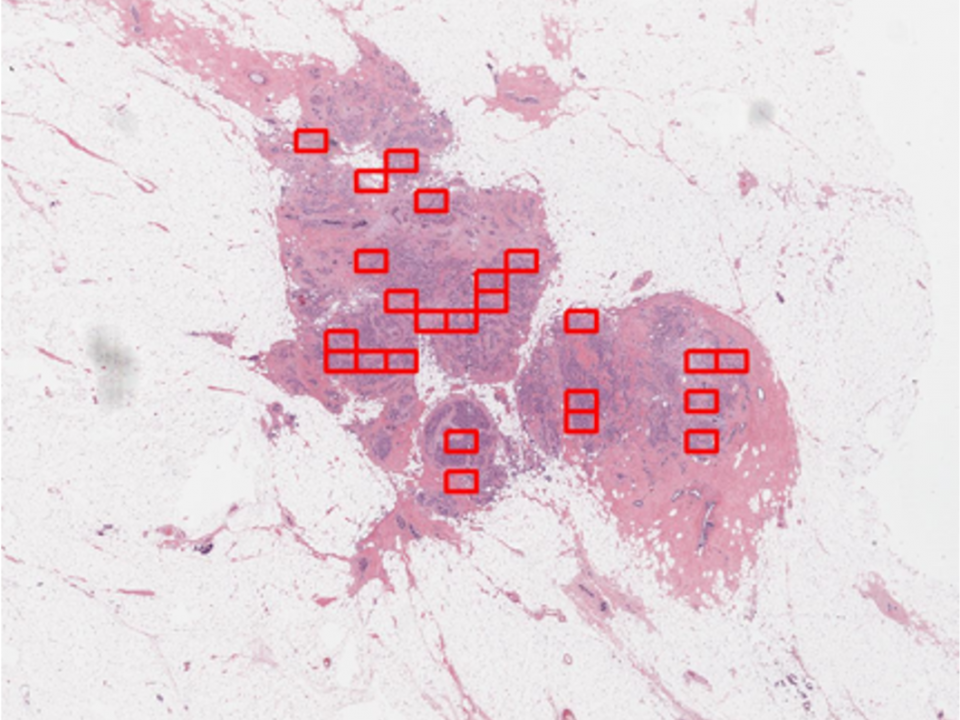
Newton, Massachusetts, November 18, 2020 — 4D Path, creator of a patented computer-aided cancer diagnostic and precision oncology platform, today announced that the U.S. Food & Drug Administration (FDA) has granted the company Breakthrough Device Designation for its first software-as-a-medical-device solution. The 4D Q-plasia OncoReader Breast provides...
December 14, 2023
November 8, 2023
October 26, 2023
September 8, 2023
February 24, 2023
January 30, 2023
August 19, 2022
May 24, 2022
May 5, 2022
March 16, 2022
March 15, 2022
February 15, 2022
November 16, 2021
September 22, 2021
August 24, 2021
May 4, 2021
April 15, 2021
March 16, 2021
December 15, 2020
December 2, 2020
November 12, 2020
September 24, 2020
February 12, 2020
January 14, 2020
December 15, 2019
December 10, 2019